Bay Crossings is happy to introduce Angus MacDonald, a doctor of Engineering Physics, University of Virginia, 1969, as our new science correspondent. We regret that, in a previous article, last minute editorial changes were made which included scientific errors. We feel sure that Dr. MacDonald’s articles will help our readers understand the science behind what we see in the world around us.
Published: November, 2005
Editor’s Note:
Bay Crossings is happy to introduce Angus MacDonald, a doctor of Engineering Physics, University of Virginia, 1969, as our new science correspondent. We regret that, in a previous article, last minute editorial changes were made which included scientific errors. We feel sure that Dr. MacDonald’s articles will help our readers understand the science behind what we see in the world around us.
Dr. Angus MacDonald
Propane - The Fuel for Cars
Those of us who are old enough to remember simpler days will recall cars with no pollution control equipment whatever. The exhaust always smelled bad, smog was bad and a hose from the exhaust to inside the car was a good way to commit suicide, by carbon monoxide poisoning. Yet, at the same time, forklift trucks drove around indoors in warehouses with a propane bottle on the back without smelling bad and without polluting the indoor air.
In this article I will seek to explain, in understandable but rigorous scientific terms why this is so, and speculate on the reasons why, 50 years later, we discount this knowledge and run our cars on gasoline.
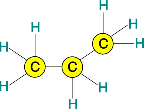
Propane is C3 H8
[See chemical diagram above]
It is a gas at ordinary room temperature yet; we can carry it around in a relatively lightweight pressure tank, because it becomes a liquid under pressure. This is a most advantageous feature. In a closed-off pressure tank, nothing boils off to create smog when the car is parked. Used in the engine, the gas mixes well with the air to form an easily burned mixture. The droplets of a liquid fuel, like gasoline, do indeed evaporate into a vapor inside the engine, but tend to create a clump of vapor improperly mixed with the air.
To understand why a propane engine burns so cleanly, a little basic science will be needed, so, here goes.
Avogadro’s Law states that equal volumes of gases contain equal numbers of molecules (about 6 x 1023 molecules per liter). The implication of Avogadro’s Law is that there will be exactly the same amount of air adjacent to each burning fuel molecule, however big the fuel molecule may be, and however much air may be needed to burn it completely.
If you take a glass jar and put it over a burning candle the flame dies down and the candle goes out and smokes. The reason for this is that the air in the jar runs out of oxygen and the nitrogen left behind puts the flame out. Air is four parts nitrogen to one part oxygen.
On a molecular level the same thing can happen.
A propane molecule is C3 H8. The reaction of carbon with oxygen is C + O2 • CO2
and with hydrogen 2H2 + O2 • 2H2O.
So, every single carbon atom in the burning molecule needs an oxygen molecule close nearby, and every four hydrogen atoms need an oxygen molecule (O2) close nearby. Counting carbons and hydrogens in propane (C3 H8), we see that 5 oxygen molecules need to be close nearby, in order to complete combustion.
If we think of molecules as little lumps all the same size (which is what Avogadro’s law says for a gas) then there will be 12 air molecules in a sphere surrounding the first one, of which three will be oxygen. One in five molecules in ordinary air is oxygen. We only need two more from a little further away, and combustion will be complete.
Experimentally, this happens.
If we think about gasoline, octane (C8 H18), it is clear that 13 oxygen molecules will be needed and there will be only three in the closest area. The area right beside the burning octane will be utterly depleted of oxygen and the surplus carbon fuel will be left to its own devices. Unfortunately, really most unfortunately in this case, carbon reacts with nitrogen under conditions of heat and pressure. Even worse, carbon nitrogen compounds tend to be rather bad for you. They use hydrogen cyanide gas (HCN) in the gas chamber at San Quentin. That is not to say that HCN is a likely outcome in this case, merely to indicate that CN compounds are the sort of thing we really don’t want around. We’d rather not make them by accident.
Remember the candle going out and smoking because there wasn’t enough air? That’s what happens in your gasoline engine, and it smells.
In your propane engine this does not happen, and it does not smell.
Fifty years ago, we knew how to end pollution by automobiles. All we needed to do was to install PCV (positive crankcase ventilation) valves and run the engines on propane. With today’s sophisticated closed loop computer controllers, we could keep the mixture perfectly accurate and build cars that run on propane and do not need catalytic converters or any other pollution gadgetry.
A purpose designed propane engine can have a higher compression ratio than our car engines, which raises the maximum temperature of combustion, and thereby raises the maximum possible thermodynamic efficiency of the engine.
In Australia there exists a network of 35,000 service stations, which dispense both petrol (gasoline) and Autogas, a mixture of propane and butane. They use propane in Australia!
I suspect that we, in the United States, took the gasoline path because our oil refineries did not want to make propane instead of gasoline and our carmakers did not want to change the cars they make. I further suspect that we, as a society, pay far more for all those catalytic converters and technological pollution fixes in all our cars than we would ever have had to spend to retool the refineries.
Rather a pity — is it not?

