 |
| Byron Anderson
is an Advanced Transportation Technology Project Coordinator at
City College of San Francisco. |
|
|
|
Types of Fuel Cells
Since the revival of interest in fuel cells several different types
have been developed. Each type has operating characteristics and
temperatures that are suitable for certain applications but not for
others.
Proton Exchange Membrane (PEM) fuel cells work with a polymer in
the form of a thin, permeable sheet coated with a TeflonTM
like material. The solid, flexible membrane will not leak or crack, and
these cells operate at a low enough temperature to make them suitable
for boats, cars and homes. But their fuels must be purified, and a
platinum catalyst is used on both sides of the membrane, raising costs.
Alkali fuel cells operate on compressed hydrogen and oxygen. They
generally use a solution of potassium hydroxide as their electrolyte.
Alkali cells were used in Apollo spacecraft to provide both electricity
and drinking water. They require pure hydrogen fuel, however, and their
platinum electrode catalysts are expensive. And like any container
filled with liquid, they can leak.
Molten Carbonate fuel cells
(MCFC) use high-temperature compounds
of salt carbonate of sodium or magnesium... Their nickel
electrode-catalysts are inexpensive compared to the platinum used in
other cells. But the high temperature also limits the materials and safe
uses of MCFCs—they would probably be too hot for home use.
Phosphoric Acid fuel cells (PAFC) use phosphoric acid as the
electrolyte.. PAFCs tolerate a carbon monoxide (a poison for other fuel
cells) in concentrations of about 1.5 percent, which broadens the choice
of fuels they can use. Platinum electrode-catalysts are needed, and
internal parts must be able to withstand the corrosive acid.
Solid Oxide fuel cells (SOFC) use a hard, ceramic compound of
metal oxides of calcium or zirconium as electrolyte. At such high
temperatures a fuel reformer is not required to extract hydrogen from
the fuel, and waste heat can be recycled to make additional electricity.
However, the high temperature limits applications of SOFC units and they
tend to be rather large.
|
Fuel Cells to the Rescue
By Byron Anderson
In a not too distant
future, sleek, quiet ferries will speed across the waters of San
Francisco Bay powered by hydrogen fuel cells. In this future, the black
smoke and the rumbling vibration of diesel powered ferries have been
relegated to quaint memories of the past, the same way we now view steam
ships or paddle wheeled boats. The ‘California energy crisis’ will
have faded from view as a distant episode of the ‘hydrocarbon age.’
As passengers traverse the white caps, some will work on laptop
computers while others talk on cell phones or contemplate a weekend trip
in their new car; all powered by fuel cells. Homes of the future will
incorporate hydrogen fuel cell technology to generate their own power.
The day may come when no one pays utility bills!
Does that seem too
visionary a picture? Actually, all the elements needed to make this
picture a reality are in development right now. The technology, though
moving rapidly, seemingly remains low on the radar screen for the
general public. For instance, readers of Bay Crossings may be
surprised to know that fuel cell powered water transit has already been
demonstrated on San Francisco Bay.
On October 2nd 2001, an
otherwise conventional 14’ runabout equipped with a prototype fuel
cell gently ‘ferried’ members of the press and the Water Transit
Authority around China Basin for a day of excursions and animated
technology discussions. The product used to power the boat was an
EnableTM hydrogen PEM fuel cell capable of generating 1 kW continuously
with peak power of nearly 2kW. This demonstration was hosted by DCH
Technology, Inc. of Valencia California, a company known for its
hydrogen sensing equipment. Arron Rachlin, company spokesman, explained
that the event was one of several demonstrations DCH was engaged in
through a grant from the California Air Resources Board. The state is in
fact promoting a host of energy related studies and demonstration
projects that involve hydrogen and fuel cell technology.
Today companies like
Ballard are developing the latest generation of fuel cell engines for
buses and cars while Fuel Cell Energy, Inc. and others put finishing
touches on fuel cell based stationary power systems. Even Coleman, Inc.
venerable makers of camping equipment, plans to produce a portable fuel
cell within a year.
Fuel Cell Origins
Despite their modern
high-tech aura, fuel cells actually have been known to science for more
than 100 years. Though generally considered a curiosity in the 1800s,
fuel cells have become the subject of intense research and development,
especially since World War II.
In 1839, William Grove
demonstrated the world’s first ‘fuel cell’ (or ‘gas battery’
as he called it) at the Royal Institution in London. Grove, a friend of
Michael Faraday, had been experimenting with electrolysis, the process
of putting an electrical current through a fluid, in this case sulfuric
acid. What surprised him was that when he disconnected the apparatus it
seemed to work backwards and he observed that he was now generating a
small voltage. Unfortunately the materials that Grove used were unstable
and public interest dwindled. It wasn’t until the 1960’s that fuel
cells were revived for use on manned space flights. NASA developed fuel
cells as the ideal supply of both power and drinking water.
Fuel Cells
for the Uninitiated
For those not up to speed
on what fuel cells are or why they are the ‘darling technology’ of
choice for our transportation-energy future, let’s briefly examine how
they work; the benefits they will bestow and some of the technology
hurdles that must be overcome.
"I believe that water
will one day be employed as a fuel, that hydrogen and oxygen will
constitute it, used singly or together will furnish an inexhaustible
source of heat and light of much greater power than coal possesses.
Bunkers, ships and locomotive tenders will store these two condensed
gases instead of coal, and they will burn in the boilers producing
enormous heat…." Jules Verne, ‘Mysterious Island’ - 1870
Everyone is familiar with
batteries, every car has one. A car battery has several cells that store
an electrical charge. Combined they deliver 12 volts of direct current
(DC). The difference between a battery and a fuel cell is that whereas
batteries store electricity, a fuel cell generates
electricity. Electricity from a fuel cell is generated by the
electro-chemical reaction taking place between two gases, hydrogen and
oxygen. The key to understanding the source of energy generated in a
fuel cell is that the hydrogen - oxygen reaction can be ‘intercepted’
to capture small amounts of electricity. The byproduct of this reaction
is the formation of water (H2O).
Application
to Marine Vessels
At a recent board meeting
of the Water Transit Authority, Bob Beadell, Business Development
Manager of Zemar Ltd. explained the plans his company has for developing
marine fuel cells. "Our focus for marine applications is currently
on what we call "captive fleets". These are vessels which have
a defined duty cycle which brings them back to their home port or berth
at the end of each day, this includes such things as small ferries and
water taxis. The reason for this is to allow them to refuel daily or as
needed. As technology develops to allow greater volumes of fuel to be
stored, without a weight or space penalty, then we shall begin to shift
our focus to include offshore marine applications."
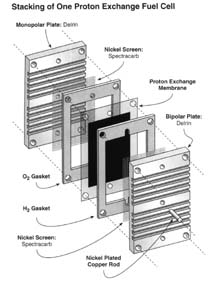 |
|
A Proton Exchange Membrane type fuel cell (PEM). The plates with
grooves channel hydrogen and oxygen on either side of the membrane.
Hydrogen and oxygen circulate and are exposed to the surface of the PEM.
In the exchanges of gases, protons pass through the membrane while
electrons are captured by the nickel screen and conducted to an external
circuit to perform work. Used by permission of Home Power Magazine
Oct.-Nov. 2001 |
In speaking of the near
term future of the technology Beadell said, "Most of these (fuel
cells) are currently very expensive but all of the manufacturers expect
costs to fall rapidly once they are mass produced….we expect even
greater cost reductions due to the elimination of expensive catalysts
and improvements in power density. This, taken into consideration with
greatly reduced maintenance costs, will make Alkaline fuel cells very
competitive with diesel or gas engines." Zemar intends to return to
the Bay Area early next year to demonstrate its alkaline fuel cell
technology in a marine application.
As Renewable as Rain
One great appeal of fuel
cells, as mentioned, is that they generate electricity with no pollution
and few if any moving parts. The hydrogen and oxygen used in generating
electricity through this method ultimately combine to form water as a
byproduct. Since fuel cells use hydrogen, one line of reasoning taken by
economists and technologists envisions our whole economy based on
hydrogen. This scenario, in which hydrogen is as renewable as rain, has
been referred to as the "hydrogen economy." A stable energy
cost is a must for economic productivity. A decrease in the cost of
energy will have a marked positive effect on the economy just as every
Californian knows firsthand what the reverse brings.
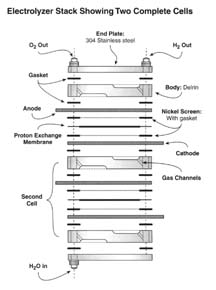 |
|
A fuel cell stack made up of two cells using the same components. In
practice, many fuel cells are assembled into a ‘stack’ of cells
(think of the several cells in your battery) which can be added upon to
attain the required current. Used by permission of Home Power Magazine
Oct.-Nov. 2001
In these two Figures we are looking at a Proton Exchange type fuel
cell (PEM). There are however, different kinds of fuel cells.
|
Technology and
Infrastructure Challenges
There are several
technological hurdles that must be overcome. Materials are expensive
right now. Platinum is used in fuel cells as a catalyst but there are
many promising materials that are less expensive being looked at.
Hydrogen storage has also been a historical problem because of low
density of the gas.
Robert Hayden of the
California Fuel Cell Partnership in West Sacramento recently wrote,
"The technological development of fuel cells as electricity
generators for transportation and stationary sources is complex. Equally
daunting is the matter of fueling. Where are we going to get the
hydrogen needed to power fuel cells? How are we going to put the
infrastructure in place that will make it as convenient and safe for
consumers to get hydrogen as it is today to get gasoline and other
fuels?"
From the standpoint of
availability, the good news is that hydrogen is relatively easy to
obtain from a number of sources and processes. Fossil fuel resellers
need not fear because there are many R & D efforts underway to find
the best ways to reform gasoline, natural gas, diesel and even coal.
The purest ideal though,
is to derive hydrogen from only renewable resources. The electrolysis of
water by use of renewable energy such as solar or wind is the model most
often mentioned. There is nothing to prevent such a plan from being put
in place except the will to do so.
Fortunately, for us, the
state of California is very interested in encouraging the development of
alternative energy including fuel cells. "Widespread use of fuel
cells in water craft would certainly help California’s efforts to
reduce air pollution," said Dr. Alan Lloyd, Chairman of the
California Air Resources Board in a recent press statement. The state
department of energy has just recently announced a Request for Proposal
(RFP) to study the whole issue of developing a hydrogen-refueling
infrastructure for fuel cell vehicles and vessels. The study will run
two years and will be published in a series of reports. The
recommendations of the final report may very well facilitate the future
mentioned at the top of the article.
Byron Anderson is an
Advanced Transportation Technology Project Coordinator at City College
of San Francisco. He has written articles for "Hydrogen
Today", "Advanced Transportation Technology" and is
webmaster and ‘AnswerGuy’ for www.clean-air.org. On the
weekends he teaches a course on electric vehicles and hydrogen fuel
cells at Sacramento City College.For more information, including details
about different types of fuel cells, see www.baycrossings.com.